N95 respirators: the gold standard in respiratory protection
Since the beginning of the pandemic, healthcare agencies like the WHO, the CDC and Health Canada have recommended that healthcare workers and other medical first responders wear certified respirators to protect themselves against COVID-19.
N95 respirators are considered the gold standard in personal protective equipment (PPE) because they filter out at 95% of small particles that include bacteria and viruses. Health Canada has created an equivalent standard called 95PFE-L2 that are certified by the CSA. The filter works by trapping bacteria and viruses before they pass through the mask, protecting both wearers and those around them. However, only an authentic respirator that meets specific standards of NIOSH and/or CSA can ensure this level of protection, whereas a fake respirator may not filter out airborne particles effectively, potentially putting the wearer at significant risk.
As demand for certified respirators increased, counterfeit masks have flooded the market. In fact, in 2020, US Customs and Border Protection (CBP) seized over 14.6 million counterfeit respirators entering the US.1

How to spot a fake N95 type respirator
Before you buy
Counterfeit N95s are respirators that are falsely marketed as being NIOSH-approved. These masks may not provide adequate respiratory protection and therefore, should be avoided. To determine whether an N95 respirator is fake or not, NIOSH markings are key, but according to CDC guidance, there are a few ways to avoid getting stuck with counterfeit respirators even before you place your order:
When buying respirators online directly through a website:
- Is the brand listed on the CDC approved respirator list or in the Health Canada Guidance document?
- Is the primary contact email address connected to the website or is it a free email account?
- Is the website content filled with typos, bad grammar, dummy text, blank pages, broken links and other errors?
- Does the site contain incorrect names or logos or misspellings in the domain name?
All of these are red flags that might indicate that you are not dealing with a reputable company.
When buying respirators through a third-party marketplace:
- If a listing has to claim that its product is “legitimate” or “genuine,” it probably isn’t.
- Check the product and seller reviews for negative feedback.
- Is the seller marketing the same products over time, or do they cater to trends? Legitimate businesses and distributors typically list a consistent offering over time.
- Does it seem too good to be true? If it does, it probably is.
- Check the quantity a seller has in stock. If the seller is advertising “unlimited stock” in the midst of a shortage, this could be an indication that the product is not authentic.
- Does the seller break marketplace policy by displaying their own contact information within images? A legitimate business would not try to circumvent marketplace policy.
After you buy
How to check if your N95 is real
If you’ve already received the respirators you ordered from a website or marketplace, how can you tell if they are legitimate products?
First rule of thumb: No NIOSH markings means no approval.
The most important aspect of determining whether your respirator is a reliable N95 respirator is National Institute for Occupational Safety and Health (NIOSH) approval. NIOSH is a section of the U.S. Centers for Disease Control and Prevention that focuses on worker health and safety. For an N95 respirator to receive NIOSH approval, it must filter at least 95% of airborne particles.2
All NIOSH-approved N95 respirators are required to have the following markings:
- Name of approval holder/manufacturer business name, a registered trademark, or an easily understood abbreviation of the applicant/approval holder’s business name as recognized by NIOSH. When applicable, the name of the entity to which the FFR has been private labeled by the approval holder may replace the approval holder business name, registered trademark, or abbreviation of the approval holder business name as recognized by NIOSH.
- NIOSH in block letters or the NIOSH logo.
- NIOSH Testing and Certification approval number, e.g., TC-84A-9375.
- NIOSH filter series and filter efficiency level, e.g., N95, N99, N100, R95, P95, P99, P100.
- Model number or part number: The approval holder’s respirator model number or part number, represented by a series of numbers or alphanumeric markings, e.g., 8577 or 8577A.
This image from the CDC can help you identify your mask’s markings:3
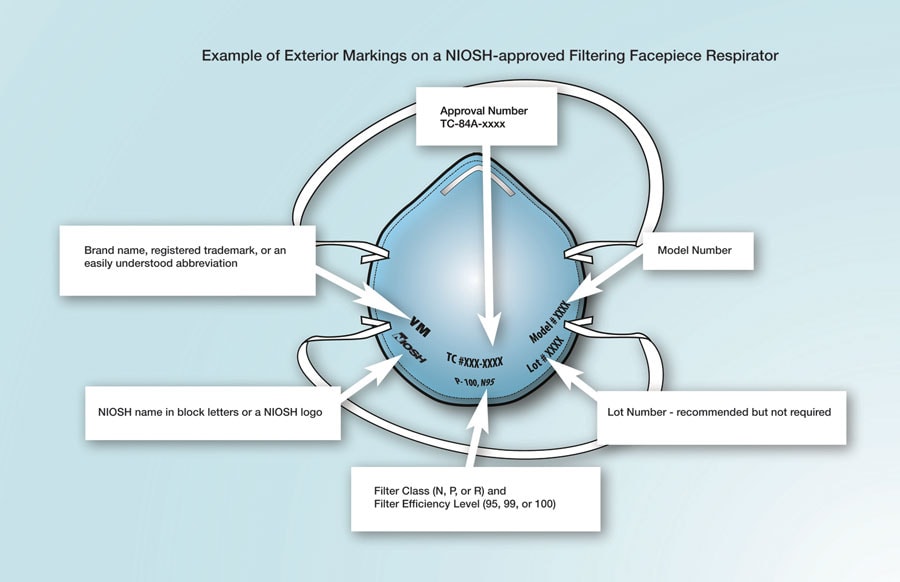
After verifying these markings, you can check for the approval number on the NIOSH certified equipment list.
Some other red flags to look out for:4
- Any decorative elements (beads, sequins, etc.)
- Earloops instead of head bands: head bands are crucial to an N95’s tight fit.
- Claims that the mask is approved for use on children (NIOSH does not approve respirators for children)
What about KN95 respirators?
It’s easy to mistake a KN95 respirator for an N95/95PFE. Although KN95s meet Chinese standards and also filter out and capture 95% of 0.3-micron particles, they are not certified by the US National Institute for Occupational Safety and Health (NIOSH).
CSA certification (Canada only)
On February 2, 2021, Health Canada announced that, as part of its Interim Order (IO) authorization process, manufacturers of filtering facepiece respirators (FFRs) must certify their products for use in Canada through an accredited certification program such as CSA Group’s new medical PPE certification program within 6 months of receiving their authorization. This applies even to manufacturers that have already been granted an IO authorization.
How to protect yourself
The best way to ensure that you’re getting the reliable protection you need is to purchase your N95s and other PPE from a well-established, reputable vendor like Medicom.
Medicom respirators
Medicom has been manufacturing and distributing high-quality PPE for over 30 years and offers NIOSH-and CSA-approved respirators and other reliable medical masks, gowns, disinfectants and more through its global network of trusted distributors.

SafeMask® Architect Pro™ Surgical N95 (US and Canada) and 95PFE-L2 Respirators (Canada)
Made in Canada, these medical grade respirators have been evaluated for use in healthcare settings according to recognized standards for biocompatibility, flammability and fluid resistance and provide optimal respiratory protection by filtering 95% of particulate aerosols free of oil.
- High-quality materials and innovative duckbill shape offer optimal breathability—no exhalation valve required.
- Unique single-split comfort bands hold respirator in place without pulling or irritating skin.
- Lightweight construction ensures comfort, even during extended wear.
- Adjustable nose piece creates a personalized fit.
- Meet N95 and fluid resistance (as per ASTM F1862 120 mm Hg) testing requirements
- Available in 3 sizes (small, medium and large)
Medicom SafeMask Architect Pro N95 surgical respirator has met the requirements of Title 42, Code of Federal Regulations, Part 84 (42 CFR 84). under approval number TC-84A-9375.


SafeMask® EdgePro™ Surgical 95PFE-L2 Respirator (Available in Canada only)
Made in Canada, this surgical respirator has been approved for use in healthcare settings. It meets all recognized standards for biocompatibility, flammability and fluid resistance and offers optimal respiratory protection by filtering 95% of particulate aerosols free of oil.
Medicom: The respirator supplier of choice for governments around the globe
Medicom has been selected by the governments of Canada, the UK, Singapore, France and Quebec to ensure local supply of respirators and other medical grade face masks and respirators. Learn more at Medicom.com.
For more information and resources related to respirators and other medical masks, please visit the Medicom Mask and Respirator Training Hub.
References
[1] https://www.cnn.com/2021/02/05/us/counterfeit-masks-how-to-spot-trnd/index.html
[2] Approved Particulate Filtering Facepiece Respirators | NPPTL | NIOSH | CDC
[3] mask-illustrationMisRep.jpg (900×582) (cdc.gov)
[4] Counterfeit Respirators / Misrepresentation of NIOSH-Approval | NPPTL | NIOSH | CDC



 English (US)
English (US)


